추천 제품
생물학적 소스
synthetic
Quality Level
분석
≥97% (HPLC)
양식
powder
불순물
glucose, essentially free
색상
white
solubility
water: slightly soluble 50 g/L
저장 온도
−20°C
SMILES string
[K+].[K+].[H]O[H].OC[C@H]1O[C@H](OP([O-])([O-])=O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13O9P.2K.H2O/c7-1-2-3(8)4(9)5(10)6(14-2)15-16(11,12)13;;;/h2-10H,1H2,(H2,11,12,13);;;1H2/q;2*+1;/p-2/t2-,3-,4+,5-,6-;;;/m1.../s1
InChI key
VOQGDSVKCMGEFO-FBNUBEQJSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
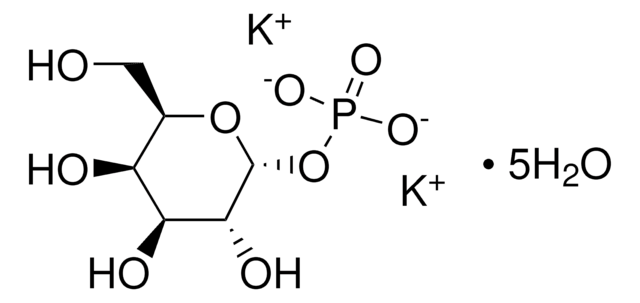
α-D-Glucose 1-phosphate is the α-anomeric form of glucose which contains a phosphate group on the primary carbon. It can be converted into the deoxysugar CDP-glucose by the enzyme α-D-Glucose-1-phosphate cytidylyltransferase.
결합
Formerly listed as Grade I.
제조 메모
Prepared by a modification of the procedure of McCready, R.M., et al., J. Am. Chem. Soc., 66, 560 (1944).
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Joerg Fettke et al.
Plant physiology, 155(4), 1723-1734 (2010-12-01)
Almost all glucosyl transfer reactions rely on glucose-1-phosphate (Glc-1-P) that either immediately acts as glucosyl donor or as substrate for the synthesis of the more widely used Glc dinucleotides, ADPglucose or UDPglucose. In this communication, we have analyzed two Glc-1-P-related
Stanley A Blumenthal
Perspectives in biology and medicine, 55(2), 236-249 (2012-05-31)
In 1945, Earl Sutherland (1915-1974) [corrected] and associates began studies of the mechanism of hormone-induced glycogen breakdown in the liver. In 1956, their efforts culminated in the identification of cyclic AMP, an ancient molecule generated in many cell types in
Jef Van der Borght et al.
Biotechnology journal, 5(9), 986-993 (2010-08-28)
β-D-Glucose-1-phosphate (βGlc1P) is an efficient glucosyl donor for both enzymatic and chemical glycosylation reactions but is currently very costly and not available in large amounts. This article provides an efficient production method of βGlc1P from trehalose and phosphate using the
Kyra-Melinda Alexacou et al.
Bioorganic & medicinal chemistry, 18(22), 7911-7922 (2010-10-16)
Glycogen phosphorylase (GP) is a promising target for the treatment of type 2 diabetes. In the process of structure based drug design for GP, a group of 15 aromatic aldehyde 4-(β-d-glucopyranosyl)thiosemicarbazones have been synthesized and evaluated as inhibitors of rabbit
Bijay Singh et al.
Protein engineering, design & selection : PEDS, 25(4), 179-187 (2012-02-16)
Two similar genes, dnmL and rmbA in Streptomyces peucetius, which encode for glucose-1-phosphate (G-1-P) thymidylyltransferases were expressed in Escherichia coli under similar conditions. While RmbA was expressed in soluble form, DnmL was found as insoluble aggregates in inclusion bodies. The
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.