21604
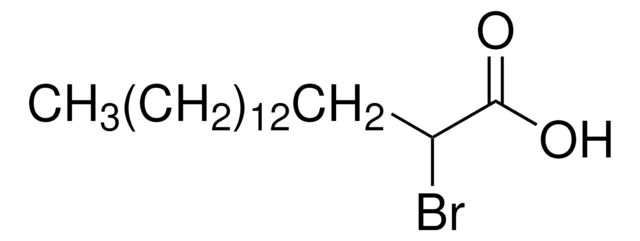
2-Bromohexadecanoic acid
≥99.0% (GC)
Synonym(s):
2-Bromopalmitic acid
About This Item
Recommended Products
assay
≥99.0% (GC)
form
solid
mp
52-54 °C
solubility
methanol: soluble 1 g/10 mL, clear, colorless
chloroform: soluble
ethanol: soluble
water: insoluble
functional group
bromo
carboxylic acid
SMILES string
CCCCCCCCCCCCCCC(Br)C(O)=O
InChI
1S/C16H31BrO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15(17)16(18)19/h15H,2-14H2,1H3,(H,18,19)
InChI key
DPRAYRYQQAXQPE-UHFFFAOYSA-N
Gene Information
human ... PPARD(5467)
Looking for similar products? Visit Product Comparison Guide
General description
Application
<li><strong>Chemo-immunotherapy Applications:</strong> 2-Bromohexadecanoic acid has been utilized to create bioactive fatty acid analog-derived hybrid nanoparticles for chemo-immunotherapy against carcinoma, demonstrating potential in cancer treatment without the need for antibodies (Tan et al., 2023).</li>
<li><strong>Drug Target Identification for Coronavirus:</strong> 2-bromopalmitate (2-BP) can be used as an inhibitor and is used to suppressed SADS-CoV treatment (Luo et al., 2021).</li>
<li><strong>Cancer Therapy Enhancement:</strong> This compound has shown efficacy in sensitizing osteosarcoma cells to adriamycin-induced apoptosis through modulation of CHOP, indicating its therapeutic potential in enhancing cancer treatment (Xu et al., 2019).</li>
<li><strong>Membrane Protein Localization in Sertoli Cells: </strong>It has been involved in studies related to the localization of the androgen receptor to plasma membranes by binding to caveolin-1 in mouse Sertoli cells, contributing to the understanding of cellular signaling pathways. It is Used to inhibit palmitoylation of androgen receptor (AR) and used to evaluate the effect of the inhibitor on androgen receptor (AR) translocation (Deng et al., 2017).</li>
<li><strong>Palmitoylation Inhibition:</strong> 2-Bromohexadecanoic acid has been profiled as an irreversible inhibitor of palmitoylation, providing insights into the regulation of protein modifications and their implications in various diseases (Davda et al., 2013).</li>
</ul>
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service