All Photos(1)
About This Item
Empirical Formula (Hill Notation):
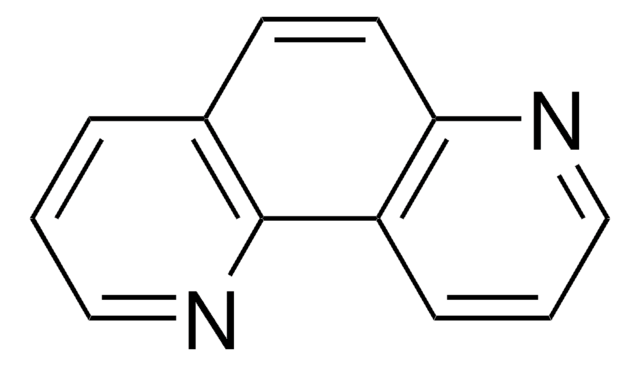
C12H8N2
CAS Number:
Molecular Weight:
180.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
172-174 °C (lit.)
SMILES string
c1cnc2ccc3ncccc3c2c1
InChI
1S/C12H8N2/c1-3-9-10-4-2-8-14-12(10)6-5-11(9)13-7-1/h1-8H
InChI key
DATYUTWESAKQQM-UHFFFAOYSA-N
General description
4,7-Phenanthroline reacts with ruthenium carbonyl to yield cyclometalated derivatives.
Application
4,7-Phenanthroline was used in preparation of:
- cyclic tetranuclear half-sandwich ruthenium(II) complexes
- positively charged homochiral cyclic trinuclear metallacalix[3]arene species
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Olsen et al.
Environmental health perspectives, 102(5), 454-458 (1994-05-01)
This study examined the changes in cellular glucose uptake induced by 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD) as measured by quantification of intracellular radioactivity in the NIH 3T3 L1 preadipocyte cell line after a 30-minute incubation with the non-metabolizable radioactive analogue of glucose
Javier A Cabeza et al.
Dalton transactions (Cambridge, England : 2003), 41(24), 7249-7257 (2012-05-10)
The reactions of [Ru(3)(CO)(12)] with four aromatic diazines have been studied in THF at reflux temperature. With phthalazine (L(1)), the compound [Ru(3)(μ-κ(2)N(2)N(3)-L(1))(μ-CO)(3)(CO)(7)] (1), which contains an intact phthalazine ligand in an axial position bridging an Ru-Ru edge through both N
E Enan et al.
Reproductive toxicology (Elmsford, N.Y.), 10(3), 191-198 (1996-05-01)
This study examined the changes in cellular glucose uptake, cAMP-dependent protein kinase (PKA), and progesterone production induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in human luteinizing granulosa cells (LGCs) in culture. The role of Ah receptor on TCDD-mediated toxicity in human LGCs was
Julien Frey et al.
Journal of the American Chemical Society, 130(33), 11013-11022 (2008-07-26)
Variously substituted coordinating rigid rods have been synthesized which incorporate a central 4,7-phenanthroline nucleus attached to two 2-pyridyl groups via its 3 and 8 positions, so as to yield bis-bidentate chelates, the two-coordinating axes of the chelates being parallel to
L W Mitchell et al.
Archives of biochemistry and biophysics, 300(1), 169-177 (1993-01-01)
Porphobilinogen synthase (PBGS) is essential to all life forms; in mammals it is definitively established that Zn(II) is required for activity. The literature regarding the metal requirement for PBGS in other animals, plants, and bacteria neither establishes nor disproves a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Pyrazino[2,3-f][1,10]phenanthroline 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/226/341/31d3909e-6700-4a3e-bfb3-9ed1f6b66ee2/640/31d3909e-6700-4a3e-bfb3-9ed1f6b66ee2.png)