All Photos(2)
About This Item
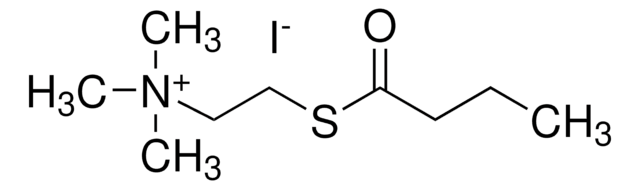
Linear Formula:
C9H20NO2Cl
CAS Number:
Molecular Weight:
209.71
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥98%
form
powder
storage temp.
−20°C
SMILES string
O.[Cl-].CCCC(=O)OCC[N+](C)(C)C
InChI
1S/C9H20NO2.ClH/c1-5-6-9(11)12-8-7-10(2,3)4;/h5-8H2,1-4H3;1H/q+1;/p-1
InChI key
VCOBYGVZILHVOO-UHFFFAOYSA-M
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Samreen Begum et al.
Computational biology and chemistry, 74, 212-217 (2018-04-14)
Amide derivatives of N-phthaloylglycine were synthesized under Schotten Baumann reaction condition. The structures of synthesized compounds (4a-d) were characterized by using FTIR, 1HNMR and EI-MS. The compounds were evaluated for their in-vitro Butyrylcholinesterase inhibition and all of them exhibited good
Babiker Elamin
Journal of biochemistry and molecular biology, 36(2), 149-153 (2003-04-12)
The dibucaine number (DN) was determined for serum cholinesterase (EC 3.1.1.8, SChE) in plasma samples. The ones with a DN of 79-82 were used, because they had the "usual" SChE variant. The enzyme was assayed colorimetrically by the reaction of
A L Gindilis et al.
Prikladnaia biokhimiia i mikrobiologiia, 34(3), 326-331 (1998-06-30)
Potentiometric choline electrodes were developed on the basis of the mediator-free bioelectrocatalysis. The electrodes made of a composite carbon-polymer material contain choline oxidase and peroxidase coimmobilized on the surface of the electrode. The rate of the potential increase was shown
Elmorsy Khaled et al.
Talanta, 83(2), 357-363 (2010-11-30)
A highly sensitive disposable screen-printed butyrylcholine (BuCh) potentiometric sensor, based on heptakis (2,3,6-tri-o-methyl)-β-cyclodextrin (β-CD) as ionophore, was developed for butyrylcholinesterase (BuChE) activity monitoring. The proposed sensors have been characterized and optimized according to the constituents of homemade printing carbon ink
M Chelminska-Bertilsson et al.
Biochimica et biophysica acta, 1202(1), 56-60 (1993-09-03)
The hydrolysis of long-chain alkanoylcholines catalyzed by butyrylcholinesterase (EC 3.1.1.8) has been studied. Radiolabelled substrates have been used and a radiochromatographic detection method developed earlier has been applied. The long-chain choline esters were found to be excellent substrates for butyrylcholinesterase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service