P1784
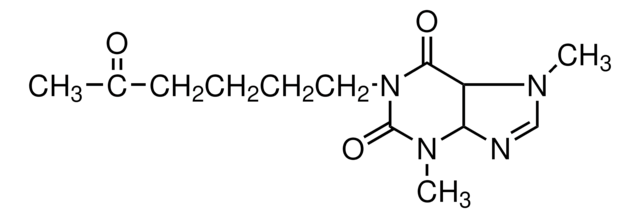
Pentoxifylline
solid
Synonym(s):
3,7-Dimethyl-1-(5-oxohexyl)xanthine, Trental
About This Item
Recommended Products
form
solid
Quality Level
color
white
solubility
H2O: ≥43 mg/mL, colorless to almost colorless
originator
Sanofi Aventis
SMILES string
CN1C=NC2C1C(=O)N(CCCCC(C)=O)C(=O)N2C
InChI
1S/C13H20N4O3/c1-9(18)6-4-5-7-17-12(19)10-11(14-8-15(10)2)16(3)13(17)20/h8,10-11H,4-7H2,1-3H3
InChI key
MQGNNJQTNFHNHK-UHFFFAOYSA-N
Gene Information
human ... ADORA2B(136) , LITAF(9516) , PDE4B(5142) , TNF(7124)
rat ... Tnf(24835)
Looking for similar products? Visit Product Comparison Guide
Application
- used in the combinatorial treatment with itraconazole for paracoccidioidomycosis (PCM)
- to treat harvested sperms to check the effect of ′pentoxifylline exposed sperms′ in the contribution of embryonic growth
- to intrathecally inject female and male mice to investigate whether astrocytes and microglia could be causally involved in the maintenance of pain-like behaviour
Biochem/physiol Actions
Features and Benefits
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Lact.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service