すべての画像(1)
About This Item
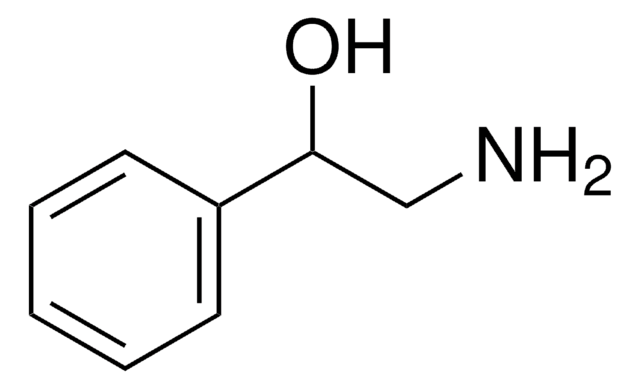
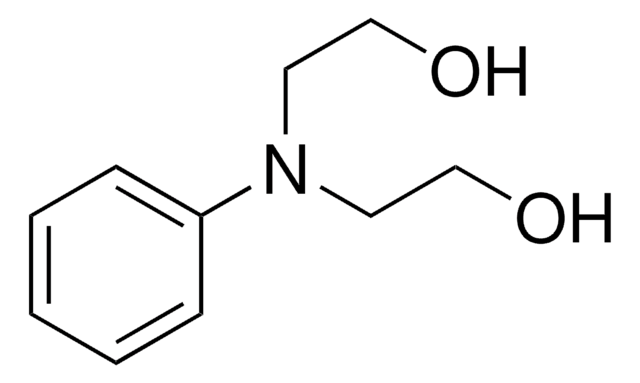
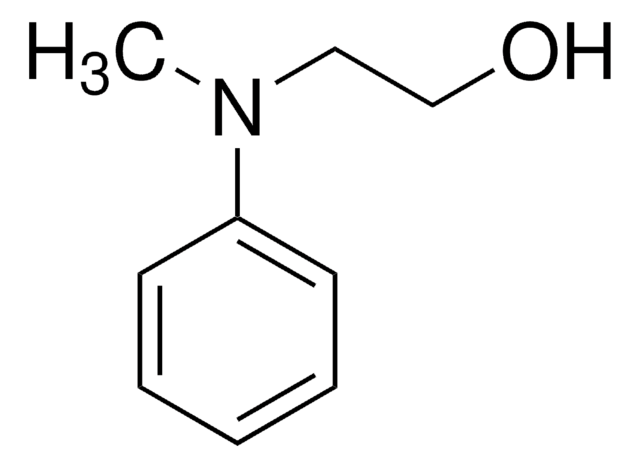
化学式:
C6H5NHCH2CH2OH
CAS番号:
分子量:
137.18
Beilstein:
774672
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
フォーム:
liquid
アッセイ:
98%
おすすめの製品
アプリケーション
N-(2-Hydroxyethyl)aniline was employed as substrate for human olfactory UDP-glucuronosyltransferase.
シグナルワード
Danger
危険有害性の分類
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - STOT RE 2 - STOT SE 1
ターゲットの組織
Blood, Blood,hematopoietic system
保管分類コード
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
引火点(°F)
235.4 °F - closed cup
引火点(℃)
113 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第三石油類
危険等級III
非水溶性液体
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
156876-100G:
156876-500G:
156876-VAR:
156876-BULK:
156876-5G:
この製品を見ている人はこちらもチェック
Santos Fustero et al.
The Journal of organic chemistry, 74(11), 4429-4432 (2009-05-15)
The preparation of cyclic dipeptide mimetics from chiral imino lactones derived from (R)-phenylglycinol is described. Key steps of the synthetic route included the fully stereoselective construction of a quaternary center, the formation of six-, seven-, or eight-membered lactams by means
Mercedes Amat et al.
The Journal of organic chemistry, 71(10), 3804-3815 (2006-05-06)
The stereochemical outcome of the alkylation of a variety of phenylglycinol-derived oxazolopiperidone lactams is studied. The influence of the configuration of the C-8a stereocenter and the effect of the substituents at the C-8 and C-8a positions on the stereoselectivity of
N Philippe et al.
Organic letters, 2(15), 2185-2187 (2000-08-10)
Highly diastereoselective protonation of chiral lactam enolates of 4-substituted-1,4-dihydroisoquinolin-3-ones is reported. Protonation and alkylation processes of these lactam enolates derived from phenylglycinol occur with opposite diastereofacial selectivity. This diastereoselective protonation has been applied to the asymmetric synthesis of (4S)-N-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline 9
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(30), 7872-7881 (2006-07-20)
A straightforward procedure for the synthesis of enantiopure polysubstituted piperidines is reported. It involves the direct generation of chiral non-racemic oxazolo[3,2-a]piperidone lactams that already incorporate carbon substituents on the heterocyclic ring and the subsequent removal of the chiral auxiliary. The
Fui-Mee Ng et al.
Molecular brain, 1, 16-16 (2008-11-20)
Phenylethanolamines selectively bind to NR2B subunit-containing N-methyl-D-aspartate-subtype of ionotropic glutamate receptors and negatively modulate receptor activity. To investigate the structural and functional properties of the ifenprodil binding domain on the NR2B protein, we have purified a soluble recombinant rat NR2B
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)