おすすめの製品
詳細
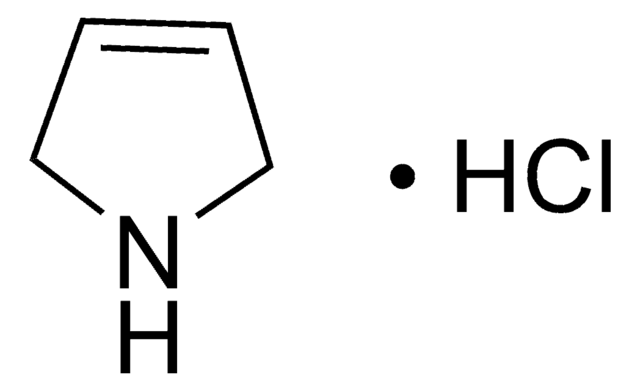
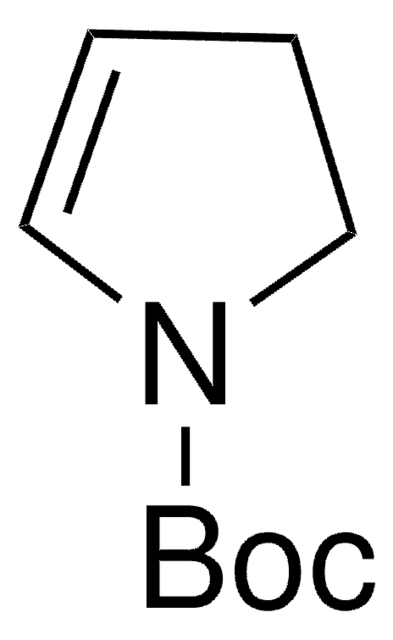
3-Pyrroline is a heterocyclic building block. The excited state dynamics of 3-pyrroline by Hamiltonian model based on the vibronic coupling model has been investigated. Trifluoromethylated azomethine ylide is reported as precursor for the synthesis of 3-pyrroline building blocks. 3-Pyrrolines are reported as highly useful intermediates for the synthesis of functionalized pyrrolines, pyrrolidines and other natural products. Preparation of 3-pyrroline(2,5-dihydro-1H-pyrrole) from (Z)-1,4-dichloro-2-butene, via Delépine Reaction has been reported. It is formed as intermediate in the synthesis of N-(tert-butyloxycarbonyl)-3-pyrroline. Reaction of Me3Al and Me3Ga with 3-pyrroline is reported.
Various 3-pyrrolines (2,5-dihydropyrroles) have been synthesized by two-step reaction sequence of alkylation/alkylidene carbene CH-insertion reaction. Synthesis of 3-pyrroline has been reported by employing cis-1,4-dichloro-2-butene as starting reagent.
アプリケーション
レニン阻害剤や血管拡張剤の合成に用いられています。
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1A
保管分類コード
3 - Flammable liquids
WGK
WGK 2
引火点(°F)
-0.4 °F - closed cup
引火点(℃)
-18 °C - closed cup
個人用保護具 (PPE)
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第一石油類
危険等級II
非水溶性液体
Jan Code
377112-1G:
377112-VAR:
377112-BULK:
377112-250MG:
この製品を見ている人はこちらもチェック
S P Neville et al.
The journal of physical chemistry. A, 118(51), 11975-11986 (2014-09-16)
A model Hamiltonian based on the vibronic coupling model is developed to describe the excited state dynamics of 3-pyrroline. With the use of the method of improved relaxation in conjunction with the MCTDH wavepacket propagation algorithm, vibrational eigenstates corresponding to
A modified procedure for the preparation of 2, 5-dihydropyrrole (3-pyrroline).
Warmus JS, et al.
The Journal of Organic Chemistry, 58(1), 270-271 (1993)
S H Rosenberg et al.
Journal of medicinal chemistry, 33(7), 1962-1969 (1990-07-01)
Incorporation of nonreactive polar functionalities at the C- and N-termini of renin inhibitors led to the development of a subnanomolar compound (21) with millimolar solubility. This inhibitor demonstrated excellent efficacy and a long duration of action upon intravenous administration to
A convenient route to 3-pyrroline utilizing the Delepine reaction.
Brandange S and Rodriguez B.
Synthesis, 4 , 347-348 (1988)
K Yoshino et al.
Journal of medicinal chemistry, 33(8), 2192-2196 (1990-08-01)
Structural modifications of the calcium antagonist fostedil (KB-944) and their coronary vasodilator activity are described. Amidophosphonates 4a-m, lactam amidophosphonates 7a-1, and diamide dilactam 10 were prepared, and their coronary vasodilator activity was assessed in dogs. Many compounds exhibited coronary vasodilator
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)