すべての画像(1)
About This Item
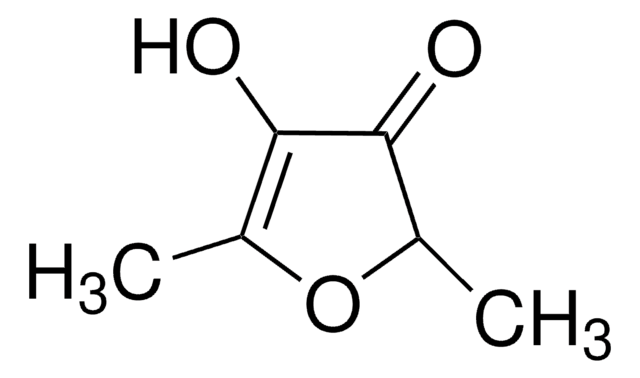
化学式:
C4OH2OCH3OH
CAS番号:
分子量:
114.10
EC Number:
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
アプリケーション
4-Hydroxy-5-methyl-3-furanone may be used in oxidoreductase assay for the evaluation of oxidoreductase activity.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
560006-5G:
560006-VAR:
560006-BULK:
Etsuko Sugawara et al.
Bioscience, biotechnology, and biochemistry, 71(7), 1761-1763 (2007-07-10)
The formation of HEMF[2(or 5)-ethyl-5(or 2)-methyl-4-hydroxy-3(2H)-furanone], the aroma component specific to miso and soy sauce, was promoted by cultivating the halo-tolerant yeast, Zygosaccharomyces rouxii, in a medium including the amino-carbonyl reaction products based on ribose and glycine. The glucose concentration
F B Whitfield et al.
Journal of agricultural and food chemistry, 49(2), 816-822 (2001-03-23)
The reaction of 4-hydroxy-5-methyl-3(2H)-furanone (HMF) with cysteine or hydrogen sulfide at pH 6.5 for 60 min at 140 degrees C produced complex mixtures of volatile compounds, the majority of these containing either sulfur or nitrogen. Of the 68 compounds detected
Hyo Jung Kim et al.
Journal of cosmetic science, 59(2), 117-125 (2008-04-15)
In previous studies, 4-hydroxy-5-methyl-3[2H]-furanone (HMF) was shown to have potent antioxidative and antimelanogenic effects, suggesting its potential use as a depigmenting agent. The present study investigated its mechanism of action on murine melanoma B16F10 cells stimulated by theophylline, an activator
Doris Marko et al.
Chemical research in toxicology, 16(1), 48-55 (2003-04-16)
We investigated the effect of a series of Maillard reaction products formed from carbohydrates under household heating conditions on the growth of human tumor cells in vitro. 4-Hydroxy-5-methyl-3-(2H)-furanone (1) was found to potently enhance the proliferation of human tumor cells.
Reinvestigation of the reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone.
A Ravagli et al.
Journal of agricultural and food chemistry, 47(12), 4962-4969 (1999-12-22)
The reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone was reinvestigated as a part of a systematic study on low molecular weight colored compounds from the Maillard reaction. In acetic acid/piperidine, besides 2-(2-furanylmethylene)-4-hydroxy-5-methyl-3(2H)-furanone (1) and 5-[2-(2-furanyl)ethenyl]-2-(2-furanylmethylene)-4-hydroxy-5-methyl -3( 2H)-furanone (2), four novel compounds, 15a
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)