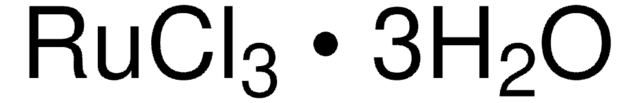おすすめの製品
品質水準
アッセイ
40-50% Ru basis (gravimetric)
99.99% trace metals basis
形状
powder
specific gravity measuring range
6.97 g/mL
不純物
≤150 ppm (trace metals analysis)
色
dark gray to black
pH
1-2
溶解性
water: soluble
密度
3.11 g/mL at 25 °C (lit.)
SMILES記法
Cl[Ru](Cl)Cl
InChI
1S/3ClH.Ru/h3*1H;/q;;;+3/p-3
InChI Key
YBCAZPLXEGKKFM-UHFFFAOYSA-K
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Ruthenium chloride is a dark brown or black solid often used as a powder. It is slightly soluble in organic solvents. Typically, ruthenium metal powder and chlorine are heated to create anhydrous ruthenium(III) chloride.
アプリケーション
Ruthenium chloride is most used as a precursor for the synthesis of ruthenium complexes. One common application of ruthenium trichloride is in the synthesis of ruthenium nanoparticles, which are used as catalysts or composited with other materials and used as co-catalysts for both oxygen and hydrogen evolution reactions Researchers have used our ruthenium chloride to produce high-quality, catalytically active ruthenium nanoparticles and ruthenium oxide nanoparticles. In addition, common application of ruthenium chloride anhydrous is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
保管分類コード
8A - Combustible corrosive hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
935484-10G:
935484-2G:
935484-BULK:
935484-VAR:
最新バージョンのいずれかを選択してください:
Nitrogen Fixation by Ru Single-Atom Electrocatalytic Reduction
Tao H, et al.
Chem, 5, 204-214 (2019)
Fabing Su et al.
Journal of the American Chemical Society, 129(46), 14213-14223 (2007-11-02)
We report here a thermal reduction method for preparing Ru catalysts supported on a carbon substrate. Mesoporous SBA-15 silica, surface-carbon-coated SBA-15, templated mesoporous carbon, activated carbon, and carbon black with different pore structures and compositions were employed as catalyst supports
Tailoring the composition of ultrathin, ternary alloy PtRuFe nanowires for the methanol oxidation reaction and formic acid oxidation reaction
Scofield M E, et al.
Energy & Environmental Science, 8, 350-363 (2015)
Youngmin Lee et al.
The journal of physical chemistry letters, 3(3), 399-404 (2012-02-02)
The activities of the oxygen evolution reaction (OER) on iridium-oxide- and ruthenium-oxide-based catalysts are among the highest known to date. However, the OER activities of thermodynamically stable rutile iridium oxide (r-IrO2) and rutile iridium oxide (r-RuO2), normalized to catalyst mass
Vinoth Ramalingam et al.
Advanced materials (Deerfield Beach, Fla.), 31(48), e1903841-e1903841 (2019-10-18)
A titanium carbide (Ti3 C2 Tx ) MXene is employed as an efficient solid support to host a nitrogen (N) and sulfur (S) coordinated ruthenium single atom (RuSA ) catalyst, which displays superior activity toward the hydrogen evolution reaction (HER).
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)