TZHALV205
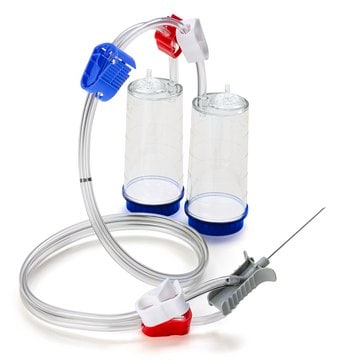
Steritest® NEO Device
For liquids in large vials., Blue base canister with a vented double needle for large glass containers with septa., pkg of 10 blisters in 2 bags of 5 blisters per box, Double packed
別名:
Blue Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
おすすめの製品
物質
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
mixed cellulose esters (MCE) membrane
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
品質水準
認証
EP (2.6.1)
JP (4.06)
USP 71
無菌性
sterile; γ-irradiated
特徴
Blue base canister with a vented double needle for large glass containers with septa.
メーカー/製品名
Steritest®
包装
pkg of 10 blisters in 2 bags of 5 blisters per box, Double packed
パラメーター
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
チューブの長さ
850 mm
色
blue Canister Base
Matrix
MF-Millipore™
ポアサイズ
0.45 μm pore size
入力
liquid
sample type pharmaceutical(s)
アプリケーション
pharmaceutical
sterility testing
適合性
For liquids in large vials.
輸送温度
ambient
関連するカテゴリー
詳細
Steritest® NEO Device is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system minimizes false positives and offers the highest levels of quality and reliability. This device ensures that pharmaceutical products are never exposed to the environment during the testing process. This test system offers an optimized and fully regulatory compliant testing process, when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device is gamma sterilized and double packed for the quick transfer into sterility testing environments, simplifying decontamination procedures and saving time. A vented needle adapter vents and transfers the test product from large volume containers with a septum to the Steritest® NEO devices. The blue based canisters indicate mixed cellulose esters (MCE) membrane, which provides an optimal filtration flow rate for standard products.
アプリケーション
特徴および利点
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
包装
法的情報
保管分類コード
11 - Combustible Solids
WGK
WGK 2
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
TZHALV205:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
資料
Sterility testing is one of the most crucial steps in pharmaceutical product release. Regulatory-compliant membrane filtration sterility testing devices ensure the safety of pharmaceutical products.
関連コンテンツ
無菌試験は、医薬品販売において最も重要なステップの1つです。規制に準拠した無菌試験用メンブレンフィルターデバイスは、医薬品の安全性を確保します。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)