おすすめの製品
由来生物
synthetic (organic)
品質水準
アッセイ
≥90% (GC)
フォーム
liquid
屈折率
n20/D 1.434 (lit.)
bp
193 °C/20 mmHg (lit.)
mp
~3 °C (lit.)
密度
0.85 g/mL at 25 °C (lit.)
官能基
ester
脂質タイプ
saturated FAs
輸送温度
ambient
保管温度
2-8°C
SMILES記法
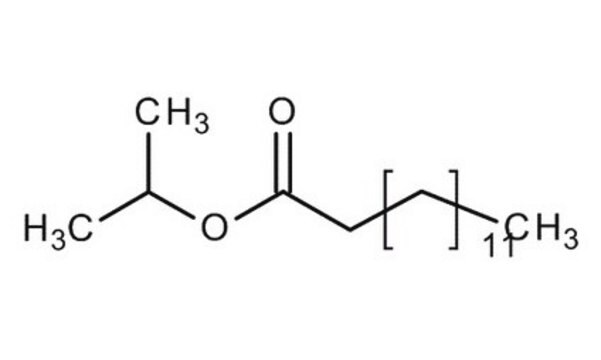
CCCCCCCCCCCCCC(=O)OC(C)C
InChI
1S/C17H34O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-17(18)19-16(2)3/h16H,4-15H2,1-3H3
InChI Key
AXISYYRBXTVTFY-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
- Formulation design and development of matrix diffusion controlled transdermal drug delivery of glimepiride.: This study discusses the design of a transdermal drug delivery system for glimepiride, utilizing matrix diffusion mechanisms potentially incorporating Isopropyl Myristate to enhance permeation (Akram et al., 2018).
- Investigation of microemulsion and microemulsion gel formulations for dermal delivery of clotrimazole.: This article evaluates formulations incorporating Isopropyl Myristate for enhanced dermal delivery of clotrimazole, highlighting its role in improving drug solubility and skin absorption rates (Zhang and Michniak-Kohn, 2018).
生物化学的/生理学的作用
Isopropyl myristate (myristic acid isopropyl ester) is used to change the physicochemical characteristics of microsheres such as poly(lactic-co-glycolic acid) (PLGA) microspheres. Isopropyl myristate is used as a oil phase component in the formulation of microemulsion systems.
保管分類コード
10 - Combustible liquids
WGK
awg
引火点(°F)
>302.0 °F
引火点(℃)
> 150 °C
個人用保護具 (PPE)
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第三石油類
危険等級III
非水溶性液体
Jan Code
M0757-1L:
M0757-BULK:
M0757-VAR:
M0757-100ML:
M0757-250ML:
この製品を見ている人はこちらもチェック
Eva Abramov et al.
Journal of colloid and interface science, 591, 363-372 (2021-02-24)
Modified microemulsions (MEs), termed by us nanodomains (NDs), seem to be suitable vehicles for dermal drug delivery due to their high surface area and the interface enriched with membrane recognizing agents, penetration enhancers, and other components. However, liquid nanodomains do
Mahendra Singh et al.
Journal of controlled release : official journal of the Controlled Release Society, 328, 895-916 (2020-10-19)
The eye is the specialized part of the body and is comprised of numerous physiological ocular barriers that limit the drug absorption at the action site. Regardless of various efforts, efficient topical ophthalmic drug delivery remains unsolved, and thus, it
Qian Zhang et al.
Pharmaceutical research, 30(1), 32-40 (2012-08-28)
In principle, maximum transepidermal fluxes of solutes should be similar for different vehicles, except when the solute or vehicle modifies the skin. Here we estimated maximum flux, stratum corneum solubility, diffusivity and permeability coefficient for a range of similarly sized
Ji Zhang et al.
International journal of pharmaceutics, 421(1), 34-44 (2011-10-01)
In this study, microemulsion microstructures, key formulation variables, and their relationship to drug transdermal permeation enhancement were investigated. A microemulsion system with high water soluble capacity was developed, using isopropyl myristate, Labrasol, and Cremophor EL as oil, surfactant, and co-surfactant
Fan Yang et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 82(1), 158-163 (2012-06-21)
Transdermal delivery of methotrexate (MTX) was investigated by using the solid-in-oil (S/O) technique. Because MTX was coated with nonionic surfactant molecules, the resulting complex was easy to dissolve in various organic solvents and provided a transparent solution in isopropyl myristate
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)