おすすめの製品
アッセイ
≥95% (HPLC)
フォーム
oil
保管条件
desiccated
under inert gas
色
colorless to yellow
保管温度
−20°C
SMILES記法
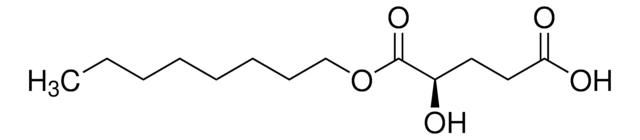
CCCCCCCCOC(C(CCC(O)=O)=O)=O
InChI
1S/C13H22O5/c1-2-3-4-5-6-7-10-18-13(17)11(14)8-9-12(15)16/h2-10H2,1H3,(H,15,16)
InChI Key
QNFIHKFBQFJVKV-UHFFFAOYSA-N
アプリケーション
Octyl-α-KG has been used to study its effects on the cell viability and to replenish α-KG levels in human glioblastoma cells.
生物化学的/生理学的作用
Octyl-α-KG (Octyl-2KG) is a membrane-permeant precursor form of α-ketoglutarate (α-KG or 2KG) whose downregulation is often seen with concomitant upregulated D-2-hydroxyglutarate (D-2HG) in tumor cells due to mutations in the NADP+-dependent isocitrate dehydrogenase genes IDH1 and IDH2, leading to reduced activity of multiple α-KG-dependent dioxygenases. Cellular α-KG delivery by Octyl-α-KG treatment (1-5 mM) is shown to restore cellular demethylase activity following octyl-2-HG (1-50 mM) treatment or IDH1(R132H) mutant expression. Octyl-α-KG also effectively reactivates α-KG-dependent dioxygenases prolyl hydroxylase (PHD) activity in cells with a dysfunctional tricarboxylic acid (TCA) cycle due to succinate dehydrogenase (SDH) and/or fumarate hydratase (FH) deficiency.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML2205-25MG:
SML2205-VAR:
SML2205-BULK:
SML2205-5MG:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
Lot/Batch Number
この製品を見ている人はこちらもチェック
Shimin Zhao et al.
Science (New York, N.Y.), 324(5924), 261-265 (2009-04-11)
Heterozygous mutations in the gene encoding isocitrate dehydrogenase-1 (IDH1) occur in certain human brain tumors, but their mechanistic role in tumor development is unknown. We have shown that tumor-derived IDH1 mutations impair the enzyme's affinity for its substrate and dominantly
Elaine D MacKenzie et al.
Molecular and cellular biology, 27(9), 3282-3289 (2007-02-28)
Succinate dehydrogenase (SDH) and fumarate hydratase (FH) are components of the tricarboxylic acid (TCA) cycle and tumor suppressors. Loss of SDH or FH induces pseudohypoxia, a major tumor-supporting event, which is the activation of hypoxia-inducible factor (HIF) under normoxia. In
Shizhong Ke et al.
Hepatology (Baltimore, Md.), 65(1), 134-151 (2016-10-25)
Erythrocytosis is a common paraneoplastic syndrome associated with hepatocellular carcinoma. Although increased erythropoietin (EPO) is found in these patients, the clinical significance and molecular mechanisms underlying this observation are unclear. We demonstrate an inverse relationship between EPO production and overall
Parker L Sulkowski et al.
Science translational medicine, 9(375) (2017-02-06)
2-Hydroxyglutarate (2HG) exists as two enantiomers, (R)-2HG and (S)-2HG, and both are implicated in tumor progression via their inhibitory effects on α-ketoglutarate (αKG)-dependent dioxygenases. The former is an oncometabolite that is induced by the neomorphic activity conferred by isocitrate dehydrogenase
Jing-Yi Chen et al.
Scientific reports, 6, 32428-32428 (2016-09-01)
Mutations of isocitrate dehydrogenase 1 (IDH1) and IDH2 in acute myeloid leukemia (AML) cells produce the oncometabolite R-2-hydroxyglutarate (R-2HG) to induce epigenetic alteration and block hematopoietic differentiation. However, the effect of R-2HG released by IDH-mutated AML cells on the bone
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)