11681460001
Roche
INT/BCIP Stock Solution
solution, pkg of 3 mL
Synonym(s):
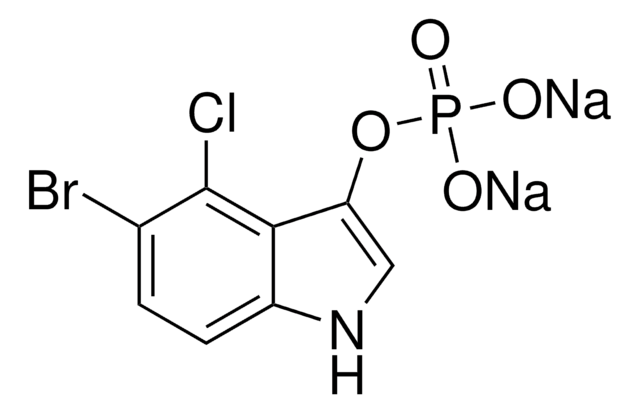
5-bromo-4-chloro-3-indoyl phosphate, BCIP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
Recommended Products
form
solution
mol wt
(INT: Mr = 505.7; BCIP toluidine salt: Mr = 433.6)
packaging
pkg of 3 mL
manufacturer/tradename
Roche
storage temp.
2-8°C
General description
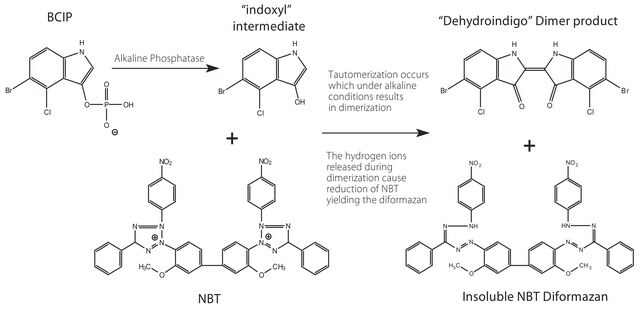
BCIP (5-bromo-4-chloro-3-indoyl phosphate) is the AP (alkaline phosphatase)-substrate which after dephosphorylation reacts further to give a dark-blue indigo dye as an oxidation product. INT (iodonitrotetrazolium) is the oxidant producing a red dye. The reaction product has a reddish-brown color and is insoluble in water.
Application
INT/BCIP Stock Solution is used for the sensitive detection of alkaline phosphatase (AP) in blotting protocols, that includes:
- Southern blot
- Western blot
- immunohistochemistry and immunocytochemistry
Specifications
Formulas: INT: C19H13CIN5O2; BCIP: C8H6NO4BrCIP x C7H9N
Principle
BCIP is the AP-substrate which after dephosphorylation reacts further to give a dark-blue indigo dye as an oxidation product. INT is the oxidant producing a red dye.
Physical form
Solution of 33 mg/ml INT (2-[4-iodophenyl]-3-[4-nitrophenyl]-5-phenyltetrazolium chloride) and 33 mg/ml BCIP (5-bromo-4-chloro-3-indolyl-phosphate, toluidine-salt in DMSO)
Preparation Note
Working solution: Preparation of 10 ml Staining Solution
Bring the stock solution to 15 to 25 °C until all components are dissolved.
Add 75 μl of the stock solution to 10 ml 0.1 M Tris-buffer, pH 9.5, 0.05 M MgCl2, 0.1 M NaCl.
Note: Prepare the staining solution shortly before use.
Preparation of Additional Solutions Required
Blocking solution: Dissolve 0.5 g Blocking Reagent in 100 ml TBS, pH 7.5, by heating to 50 to 60 °C (1 hour). Dissolving the components can be accelerated by sonication or by incubation in a microwave oven.
Information Note: The solution remains turbid.
Bring the stock solution to 15 to 25 °C until all components are dissolved.
Add 75 μl of the stock solution to 10 ml 0.1 M Tris-buffer, pH 9.5, 0.05 M MgCl2, 0.1 M NaCl.
Note: Prepare the staining solution shortly before use.
Preparation of Additional Solutions Required
Blocking solution: Dissolve 0.5 g Blocking Reagent in 100 ml TBS, pH 7.5, by heating to 50 to 60 °C (1 hour). Dissolving the components can be accelerated by sonication or by incubation in a microwave oven.
Information Note: The solution remains turbid.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 2
flash_point_f
does not flash
flash_point_c
does not flash
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Practical Methods in Cardiovascular Research
Dhein S
Springer Science & Business Media (2006)
Rahul Satija et al.
Nature biotechnology, 33(5), 495-502 (2015-04-14)
Spatial localization is a key determinant of cellular fate and behavior, but methods for spatially resolved, transcriptome-wide gene expression profiling across complex tissues are lacking. RNA staining methods assay only a small number of transcripts, whereas single-cell RNA-seq, which measures
Miao Gui et al.
Cell, 184(23), 5791-5806 (2021-10-30)
Dynein-decorated doublet microtubules (DMTs) are critical components of the oscillatory molecular machine of cilia, the axoneme, and have luminal surfaces patterned periodically by microtubule inner proteins (MIPs). Here we present an atomic model of the 48-nm repeat of a mammalian
Peichao Li et al.
Carcinogenesis, 42(1), 136-147 (2020-07-28)
Hexavalent chromium [Cr(VI)] is a potent human lung carcinogen. Multiple mechanisms have been proposed that contribute to Cr(VI)-induced lung carcinogenesis including oxidative stress, DNA damage, genomic instability and epigenetic modulation. However, the molecular mechanisms and pathways mediating Cr(VI) carcinogenicity have
Anna-Carina Weiss et al.
Development (Cambridge, England), 141(17), 3420-3430 (2014-08-21)
The vesico-ureteric junction (VUJ) forms through a complex developmental program that connects the primordium of the upper urinary tract [the nephric duct (ND)] with that of the lower urinary tract (the cloaca). The signals that orchestrate the various tissue interactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service