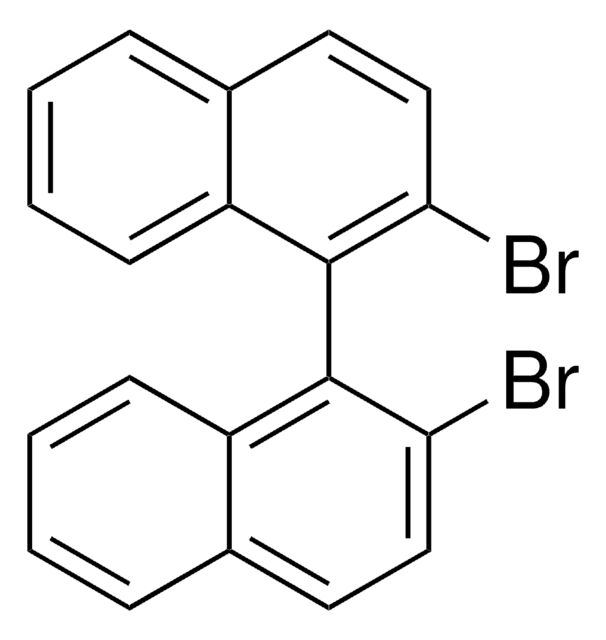
おすすめの製品
品質水準
アッセイ
99%
反応適合性
reagent type: ligand
mp
214-217 °C (lit.)
SMILES記法
Oc1ccc2ccccc2c1-c3c(O)ccc4ccccc34
InChI
1S/C20H14O2/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22H
InChI Key
PPTXVXKCQZKFBN-UHFFFAOYSA-N
アプリケーション
不斉Michael付加反応、エナンチオ選択的Diels-Alder反応、アルキニル化に用いるキラル触媒およびキラル補助基
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
104655-25G:
104655-BULK:
104655-5G:
104655-VAR:
104655-1KG:
この製品を見ている人はこちらもチェック
Yifeng Zhou et al.
Organic letters, 6(23), 4147-4149 (2004-11-05)
The readily available and inexpensive (S)-BINOL ligand in combination with Ti(O(i)Pr)(4) is an effective chiral catalyst for the catalytic asymmetric addition of alkynylzinc to unactivated simple ketones. Good to excellent enantioselectivities were achieved. No previous case has been reported successfully
Liheng Feng et al.
Organic & biomolecular chemistry, 9(8), 2938-2942 (2011-03-08)
A glucose sensing switch is formed by water soluble conjugated polymer (PP-S-BINOL) and boronic acid-functionalized benzyl viologen (o-BBV). The two-component system shows a high sensitivity for glucose sensing with a 17-fold increase in the fluorescence intensity in the presence of
Yolanda Pérez-Fuertes et al.
Nature protocols, 3(2), 210-214 (2008-02-16)
A simple three-component chiral derivatization protocol for determining the enantiopurity of chiral primary amines by 1H NMR spectroscopic analysis is described here. The method involves condensation of the amines with 2-formylphenylboronic acid and enantiopure 1,1'-bi-2-naphthol. This approach affords a mixture
Hai-Lei Cui et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(7), 1574-1577 (2009-01-10)
The first highly enantioselective allylic-allylic alkylation of alpha,alpha-dicyanoalkenes and Morita-Baylis-Hillman carbonates by dual catalysis of (DHQD)(2)AQN and (S)-BINOL has been investigated. Excellent stereoselectivities have been achieved for a broad spectrum of substrates (d.r. > 99:1, up to 99 % ee).
Junjie Ou et al.
Journal of separation science, 28(17), 2282-2287 (2005-12-14)
Two molecular imprinting polymer (MIP) monolithic columns with (S)-(-)-1,1'-bi-2-naphthol and (R)-(+)-5,5',6,6',7,7',8,8'-octahydro-1,1'-bi-2-naphthol as the templating molecules, respectively, have been prepared by in situ polymerization using 4-vinylpyridine and ethylene dimethacrylate as functional monomer and cross-linker, respectively. The columns with good flow-through properties
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)