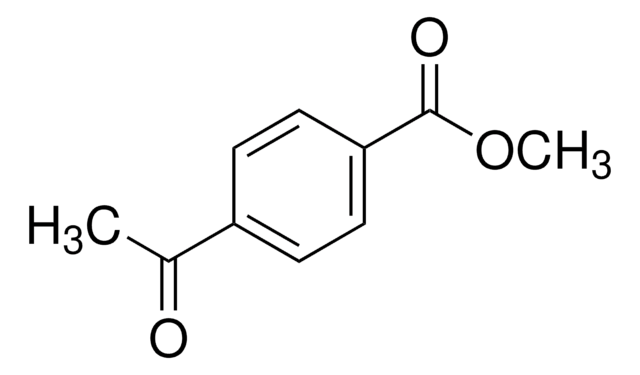
おすすめの製品
品質水準
アッセイ
≥95%
フォーム
solid
色
white to beige
保管温度
2-8°C
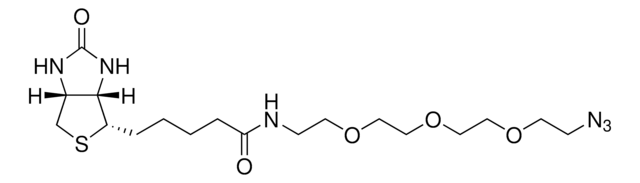
SMILES記法
[N-]=[N+]=NCCCNC(=O)CCCCC1SCC2NC(=O)NC12
InChI
InChI=1S/C13H22N6O2S/c14-19-16-7-3-6-15-11(20)5-2-1-4-10-12-9(8-22-10)17-13(21)18-12/h9-10,12H,1-8H2,(H,15,20)(H2,17,18,21)/t9-,10-,12-/m0/s1
アプリケーション
This reagent enables the specific labeling of various alkynylated molecules, such as DNA, oligonucleotides, and proteins, with biotin. The binding of biotin to avidin or streptavidin can be employed in downstream affinity applications, such as the isolation of biotinylated molecules or their interaction with streptavidin conjugates. Biotin azide undergoes a copper-catalyzed click reaction with terminal alkynes, enabling the incorporation of biotin and biotin derivatives into biomolecules that contain alkyne groups through azide-alkyne cycloaddition.
特徴および利点
Biotin-azide (N-(3-Azidopropyl)biotinamide) is an azido derived biotin probe. Biotin-azide can be used to prepare various biotinylated conjugates via Click Chemistry.The conjugation of biotin and its derivatives to various biomolecules can be achieved through the widely recognized click chemistry methodology, followed by their detection using streptavidin, avidin, or NeutrAvidin biotin-binding proteins. Biotin azide serves as a valuable reagent for the synthesis of diverse biotinylated conjugates via Click Chemistry
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
935395-VAR:
935395-25MG:
935395-100MG:
935395-BULK:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
Lot/Batch Number
Development of a Multifunctional Benzophenone Linker for Peptide Stapling and Photoaffinity Labelling
Wu Y, et al.
Chembiochem, 17, 689-692 (2016)
D C Montgomery et al.
Methods in enzymology, 574, 105-123 (2016-07-18)
Changes in reversible protein acetylation mediate many key aspects of genomic regulation and enzyme function. The catalysts for this posttranslational modification, lysine acetyltransferases (KATs), have been difficult targets for characterization due to their complex architecture and challenging reconstitution. To address
Christina M Woo et al.
Analytical and bioanalytical chemistry, 409(2), 579-588 (2016-10-04)
Protein glycosylation is a post-translational modification (PTM) responsible for many aspects of proteomic diversity and biological regulation. Assignment of intact glycan structures to specific protein attachment sites is a critical step towards elucidating the function encoded in the glycome. Previously
Kristina Hofmann et al.
Journal of lipid research, 55(3), 583-591 (2013-12-18)
Cholesterol is an important lipid of mammalian cells and plays a fundamental role in many biological processes. Its concentration in the various cellular membranes differs and is tightly regulated. Here, we present a novel alkyne cholesterol analog suitable for tracing
Qingfei Zheng et al.
The Journal of organic chemistry, 85(3), 1691-1697 (2019-12-26)
Methylglyoxal (MGO) is a reactive dicarbonyl metabolite that modifies histones in vivo and induces changes in chromatin structure and function. Here we report the synthesis and application of a chemical probe for investigating MGO-glycation. A two-step synthesis of a Cu-click
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)



![トリス[(1-ベンジル-1H-1,2,3-トリアゾール-4-イル)メチル]アミン 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)