おすすめの製品
グレード
analytical standard, for drug analysis
品質水準
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
forensics and toxicology
pharmaceutical (small molecule)
veterinary
フォーマット
neat
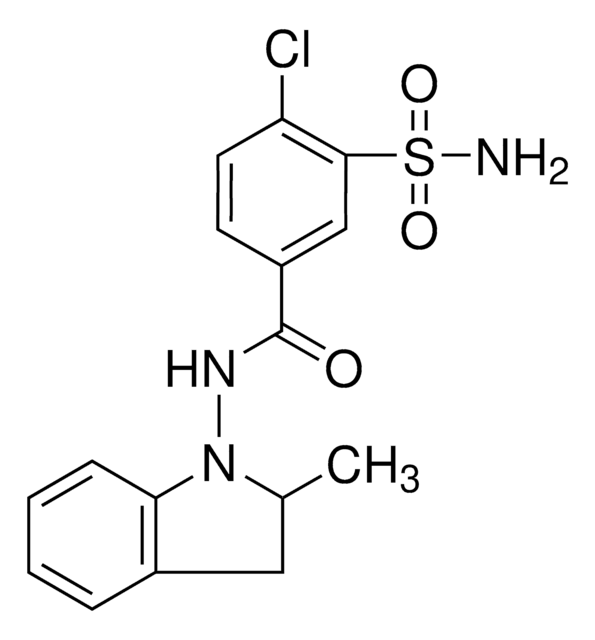
SMILES記法
CC1Cc2ccccc2N1NC(=O)c3ccc(Cl)c(c3)S(N)(=O)=O
InChI
1S/C16H16ClN3O3S/c1-10-8-11-4-2-3-5-14(11)20(10)19-16(21)12-6-7-13(17)15(9-12)24(18,22)23/h2-7,9-10H,8H2,1H3,(H,19,21)(H2,18,22,23)
InChI Key
NDDAHWYSQHTHNT-UHFFFAOYSA-N
遺伝子情報
human ... SLC12A3(6559)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Indapamide is a deuretic drug, belonging to the class of benzothiadiazines. It is commonly used for the treatment of hypertension. The drug acts by causing a drop in systolic, diastolic and mean blood pressure.
アプリケーション
Indapamide may be used as an internal standard for the quantification of the analyte in pharmaceutical formulations and biological samples using different analytical techniques
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Lact. - Repr. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
I1887-5G:
I1887-BULK:
I1887-250MG:
I1887-VAR:
I1887-1G:
この製品を見ている人はこちらもチェック
Simultaneous estimation of amlodipine besylate and indapamide in a pharmaceutical formulation by a high performance liquid chromatographic (RP-HPLC) method
Patel.BD, et al.
Scientia Pharmaceutica, 80(3), 581-590 (2012)
N Beckett et al.
BMJ (Clinical research ed.), 344, d7541-d7541 (2012-01-06)
To assess if very elderly people with hypertension obtain early benefit from antihypertensive treatment. One year open label active treatment extension of randomised controlled trial (Hypertension in the Very Elderly Trial (HYVET)). Hospital and general practice based centres mainly in
Susan van Dieren et al.
Diabetes research and clinical practice, 98(1), 83-90 (2012-06-09)
To asses differences in treatment effects of a fixed combination of perindopril-indapamide on major clinical outcomes in patients with type 2 diabetes across subgroups of cardiovascular risk. 11,140 participants with type 2 diabetes, from the ADVANCE trial, were randomized to
Alina Porfire et al.
Journal of pharmaceutical and biomedical analysis, 70, 301-309 (2012-08-14)
This paper describes the development and application of NIR-chemometric methods for active content assay and pharmaceutical characterization (granulometric analysis and flowability assessment) of indapamide powder blends for tabletting. Indapamide powder blends were prepared and their NIR spectra were recorded in
A selective HPLC method for the determination of indapamide in human whole blood: Application to a bioequivalence study in Chinese volunteers
Hang J-T, et al.
Journal of Pharmaceutical and Biomedical Analysis, 40(1), 202-205 (2006)
Chromatograms
application for HPLCapplication for HPLCライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)