905224
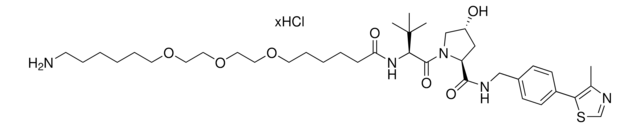
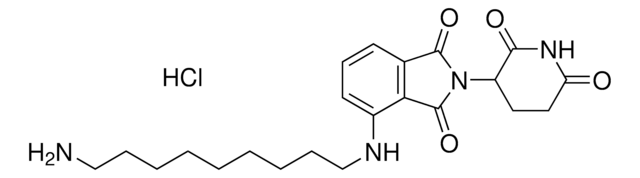
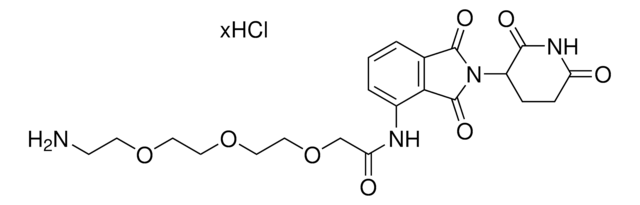
Pomalidomide-PEG6-butyl amine hydrochloride
≥95%
동의어(들):
24-Amino-N-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin, Crosslinker–E3 Ligase ligand conjugate, Pomalidomide-2-2-2-2-2-2-6-NH2 HCl salt, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
추천 제품
ligand
pomalidomide
분석
≥95%
양식
powder or crystals
반응 적합성
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
작용기
amine
저장 온도
2-8°C
SMILES string
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(COCCOCCOCCOCCOCCOCCCCCCN)=O)=O)NC1=O.Cl
InChI key
CAPZWAWDFXEZTQ-UHFFFAOYSA-N
애플리케이션
기타 정보
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
법적 정보
관련 제품
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.