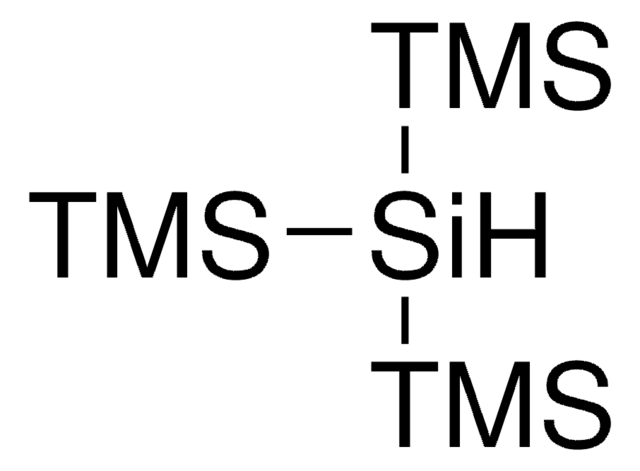おすすめの製品
形状
liquid
品質水準
反応適合性
reagent type: reductant
屈折率
n20/D 1.526
密度
0.887 g/mL at 25 °C
保管温度
2-8°C
SMILES記法
CC[Si](CC)(CC)[SiH]([Si](CC)(CC)CC)[Si](CC)(CC)CC
InChI
1S/C18H46Si4/c1-10-20(11-2,12-3)19(21(13-4,14-5)15-6)22(16-7,17-8)18-9/h19H,10-18H2,1-9H3
InChI Key
WNGZMQFMMHZKBG-UHFFFAOYSA-N
アプリケーション
Tris(triethylsilyl)silane can be incorporated as a directing group for various regio- and stereo-selective reactions. Hydrogen abstraction from tris(triethylsilyl)silane yields highly stable silyl radical.
Tris(triethylsilyl)silane can be used as a hydrogen atom donor reagent in chemical synthesis due to its weak Si-H bond. Hydrogen abstraction from tris(triethylsilyl)silane yields a highly stable silyl radical.
It can be used as a reagent:
It can be used as a reagent:
- In the radical coupling reaction to generate C-C bonds from alkyl-halogen compounds using iridium and nickel catalysts.
- To synthesize α-arylated product via cross-electrophile coupling reaction between α-chloro carbonyl and aryl bromide in the presence of nickel and iridium catalysts.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
10 - Combustible liquids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
736856-5G:
736856-VAR:
736856-1G:
736856-BULK:
この製品を見ている人はこちらもチェック
Generation of Organolithium Compounds bearing Super Silyl Ester and their Application to Matteson Rearrangement.
Oda S and Yamamoto H
Angewandte Chemie (International Edition in English), 125(31), 8323-8326 (2013)
Synthesis of ?-Hydroxy-?-haloesters through Super Silyl Ester Directed Syn-Selective Aldol Reaction.
Oda S and Yamamoto H
Organic Letters, 15(23), 6030-6033 (2013)
Selective Michael Reaction Controlled by Supersilyl Protecting Group.
Izumiseki A and Yamamoto H
Angewandte Chemie (International Edition in English), 127(30), 8821-8823 (2015)
Jonathan D Bell et al.
Chemical Society reviews, 50(17), 9540-9685 (2021-07-27)
Photoredox chemistry with organic or transition metal agents has been reviewed in earlier years, but such is the pace of progress that we will overlap very little with earlier comprehensive reviews. This review first presents an overview of the area
Tiffany Q Chen et al.
Angewandte Chemie (International ed. in English), 58(41), 14584-14588 (2019-08-15)
Here, we demonstrate that a metallaphotoredox-catalyzed cross-electrophile coupling mechanism provides a unified method for the α-arylation of diverse activated alkyl chlorides, including α-chloroketones, α-chloroesters, α-chloroamides, α-chlorocarboxylic acids, and benzylic chlorides. This strategy, which is effective for a wide variety of
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)