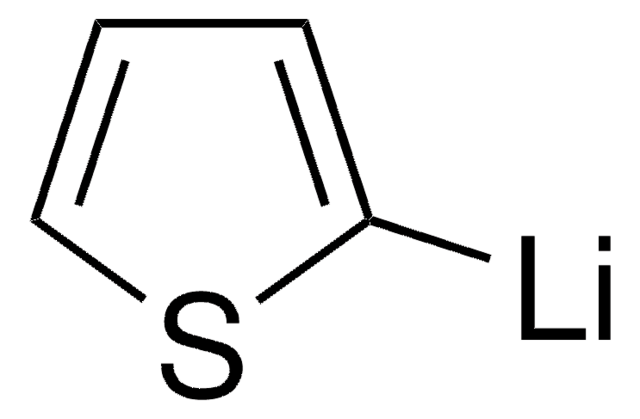
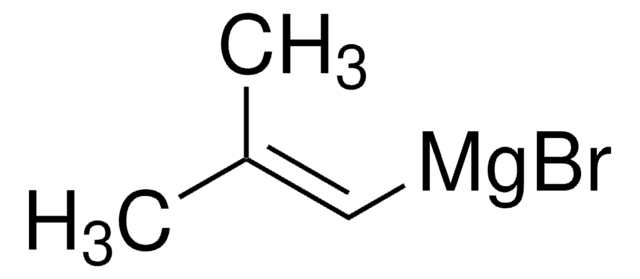
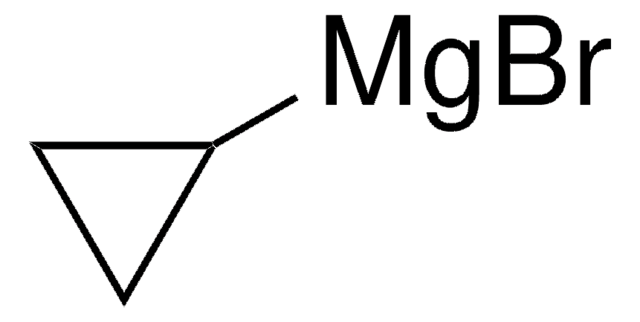
추천 제품
반응 적합성
reaction type: Grignard Reaction
Quality Level
농도
1.0 M in THF
bp
65 °C
density
1.011 g/mL at 25 °C
SMILES string
Br[Mg]c1cccs1
InChI
1S/C4H3S.BrH.Mg/c1-2-4-5-3-1;;/h1-3H;1H;/q;;+1/p-1
InChI key
GSHPYJFNTAMRJF-UHFFFAOYSA-M
애플리케이션
2-Thienylmagnesium bromide is a Grignard reagent that can be used as a reactant to synthesize:
- 1-(2-thienyl)-carbinols by condensation reaction with aldehydes. Carbinols are further dehydrated to form 2-thienyl olefins.
- Thiophene-functionalized polystyrene macromonomer (ThPStM), which is employed as a key intermediate to synthesize polystyrene-graft-polythiophene (PSt-g-PTh) via a combination of atom transfer radical polymerization (ATRP) and Grignard reaction.
- Thienylene oligomers, which are used as conducting polymers and as potential OLEDs.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-6.0 °F - closed cup
Flash Point (°C)
-21.1 °C - closed cup
이미 열람한 고객
Synthesis and characterization of 9, 9-dialkylfluorene capped benzo [c] thiophene/benzo [c] selenophene analogs as potential OLEDs
Mohanakrishnan AK, et al.
Tetrahedron Letters, 49(32), 4792-4795 (2008)
A convenient synthesis of 2, 5-thienylene oligomers; some of their spectroscopic and electrochemical properties
Van Pham C, et al.
Phosphorus, Sulfur, and Silicon and the Related Elements, 46(3-4), 153-168 (1989)
Condensations of aldehydes with 2-thienyllithium, 2-thienylsodium and 2-thienylmagnesium bromide
Van ZG, et al.
Journal of the American Chemical Society, 78(9), 1955-1958 (1956)
Atom transfer radical polymerization of MMA with a macromolecular ligand in a fluorinated solvent and in supercritical carbon dioxide.
Grignard B, et al.
European Polymer Journal, 44(3), 861-871 (2008)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![4-[Bis(trimethylsilyl)amino]phenylmagnesium bromide solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/109/860/38618a54-089d-4f50-aacc-61f10c5c12ba/640/38618a54-089d-4f50-aacc-61f10c5c12ba.png)