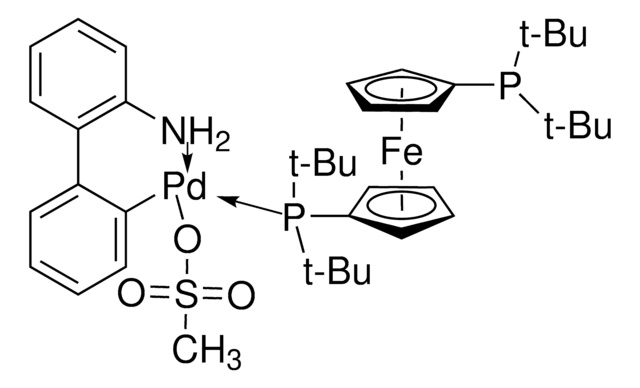
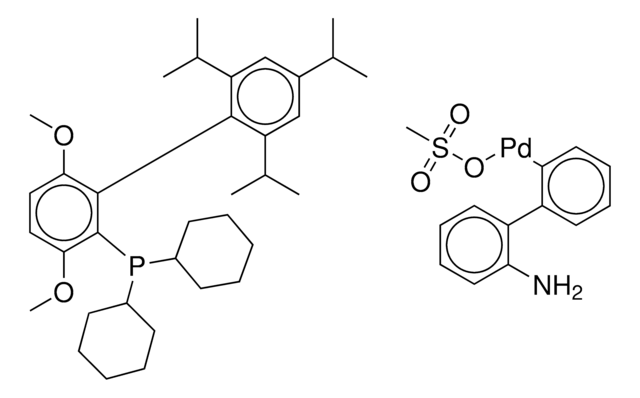
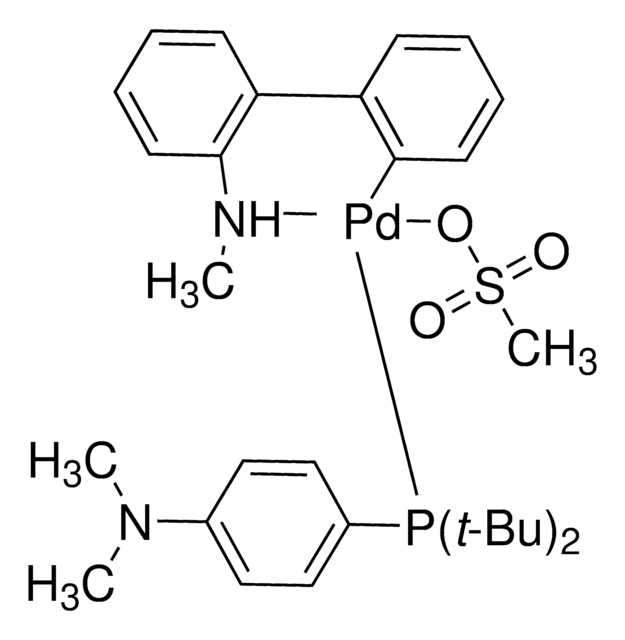
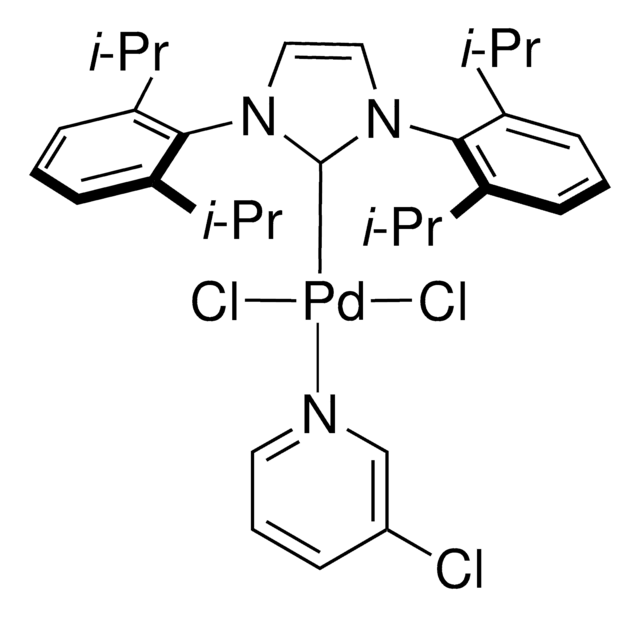
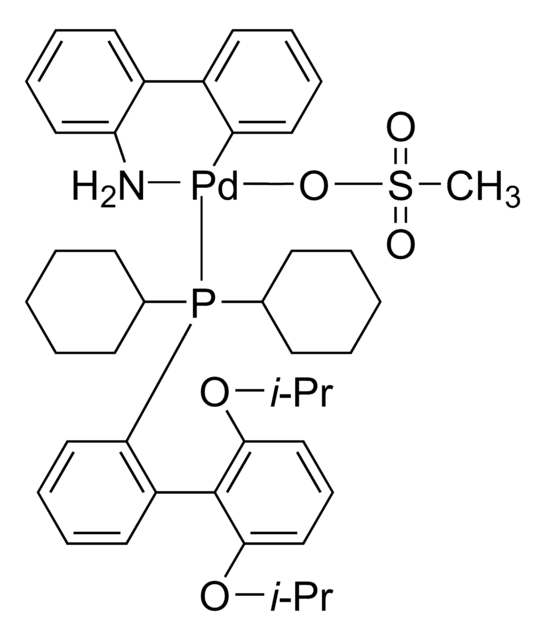
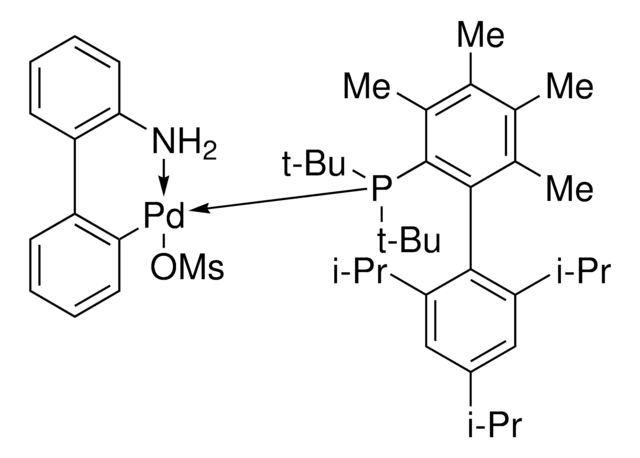
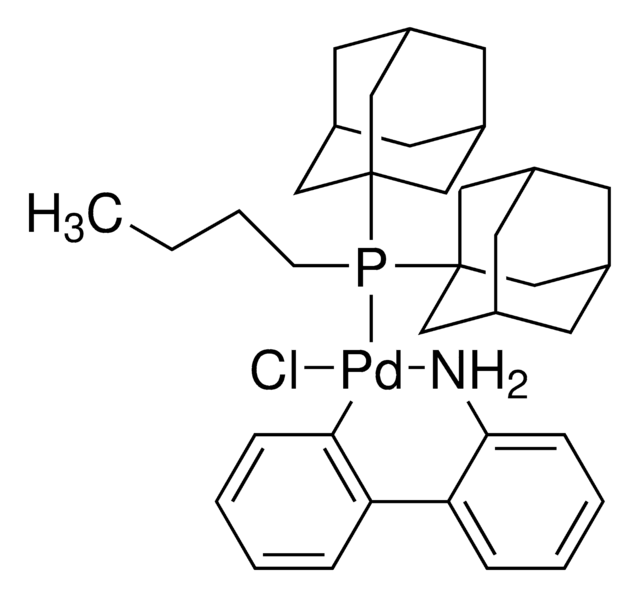
おすすめの製品
品質水準
アッセイ
97%
形状
solid
特徴
generation 3
反応適合性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
環境により配慮した代替製品スコア
old score: 5
new score: 3
Find out more about DOZN™ Scoring
環境により配慮した代替製品の特徴
Waste Prevention
Atom Economy
Less Hazardous Chemical Syntheses
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
192-201 °C (decomposition)
官能基
phosphine
環境により配慮した代替製品カテゴリ
SMILES記法
NC1=C(C=CC=C1)C2=C([Pd]OS(C)(=O)=O)C=CC=C2.CN(C)C3=CC=C(C=C3)P(C(C)(C)C)C(C)(C)C
InChI
1S/C16H28NP.C12H10N.CH4O3S.Pd/c1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h9-12H,1-8H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChI Key
SNUBBUQVCDWEAV-UHFFFAOYSA-M
詳細
アプリケーション
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
試験成績書(COA)
この製品を見ている人はこちらもチェック
資料
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Materials Included in your KITALYSIS-24PD-2PK High-Throughput Screening Kit
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
関連コンテンツ
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)