914827
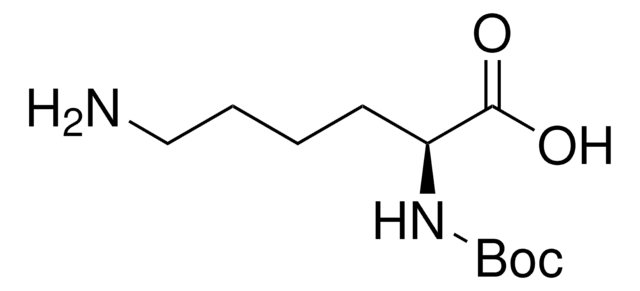
N6-((Prop-2-yn-1-yloxy)carbonyl)-L-lysine hydrochloride
≥98%
別名:
(S)-Amino-6-((prop-2-ynyloxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(Poc)-OH HCl, Lysine-alkyne, Plk, Propargyl-derivatized lysize, UAA crosslinker
ログイン組織・契約価格を表示する
すべての画像(2)
About This Item
おすすめの製品
アッセイ
≥98%
形状
powder
保管温度
2-8°C
アプリケーション
N6-((Prop-2-yn-1-yloxy)carbonyl)-L-lysine hydrochloride is a clickable amino acid derivative for site-specific incorporation into recombinant proteins or synthesis of chemical probes and tools for biological applications. This non-canonical lysine possesses an alkyne for bioorthogonal reaction with azides.
その他情報
Site-Specific Encoding of Photoactivity in Antibodies Enables Light-Mediated Antibody-Antigen Binding on Live Cells Quick View Other Sources
PEGylated polylysine derived copolymers with reduction-responsive side chains for anticancer drug delivery
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
Combined Use of Unnatural Amino Acids Enables Dual-Color Super-Resolution Imaging of Proteins via Click Chemistry
PEGylated polylysine derived copolymers with reduction-responsive side chains for anticancer drug delivery
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
Combined Use of Unnatural Amino Acids Enables Dual-Color Super-Resolution Imaging of Proteins via Click Chemistry
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
914827-250MG:
914827-BULK:
914827-VAR:
最新バージョンのいずれかを選択してください:
Andreas Schmidt et al.
ACS chemical biology, 13(9), 2472-2483 (2018-08-01)
Single-molecule techniques allow unique insights into biological systems as they provide unrivaled access to structural dynamics and conformational heterogeneity. One major bottleneck for reliable single-molecule Förster resonance energy transfer (smFRET) analysis is the identification of suitable fluorophore labeling sites that
Ivana Nikić et al.
Angewandte Chemie (International ed. in English), 53(8), 2245-2249 (2014-01-30)
The growing demands of advanced fluorescence and super-resolution microscopy benefit from the development of small and highly photostable fluorescent probes. Techniques developed to expand the genetic code permit the residue-specific encoding of unnatural amino acids (UAAs) armed with novel clickable
Yiming Li et al.
Organic & biomolecular chemistry, 11(16), 2624-2629 (2013-03-02)
Three alkyne-containing pyrrolysine derivatives were synthesized and genetically encoded into proteins by a mutant PylRS-tRNA pair with high efficiencies. With these alkyne handles, site-specific dual labeling of proteins can be achieved via a bioorthogonal thiol-yne ligation reaction.
K W Swiderska et al.
Bioorganic & medicinal chemistry, 25(14), 3685-3693 (2017-05-20)
Recent advances in site-specific protein modification include the increasingly popular incorporation of unnatural amino acid(s) using amber codon, a method developed by Schultz and coworkers. In this study, we employ this technique to introduce propargyllysine (PrK) in human fibroblast growth
Claudio Zambaldo et al.
Journal of the American Chemical Society, 139(34), 11646-11649 (2017-08-16)
Nisin is a complex lanthipeptide that has broad spectrum antibacterial activity. In efforts to broaden the structural diversity of this ribosomally synthesized lantibiotic, we now report the recombinant expression of Nisin variants that incorporate noncanonical amino acids (ncAAs) at discrete
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)